Infrastructure
Clinical Research Unit
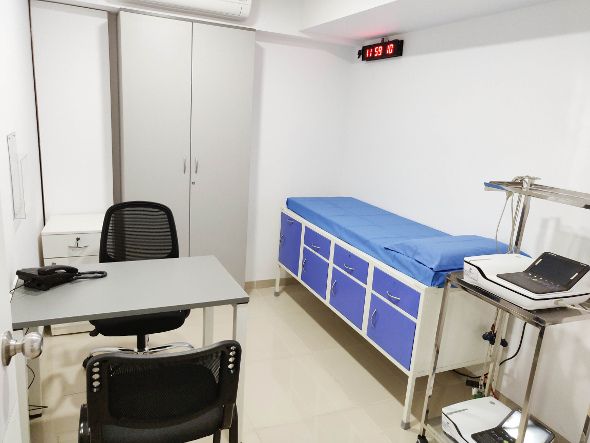
Synergen Bio has state-of-the-art infrastructure to facilitate a quality conduct of research and is a GCP compliant facility manned by an experienced team of medical professionals and supported by a team of clinical investigators, physicians, clinical study coordinators, pharmacists and freezer custodians. A well equipped ICU with the entire necessary infrastructure to support 160 beds for all clinical pharmacology studies. The facility is designed to meet Global Regulatory Standards. The main components of infrastructure at Synergen Bio are well equipped facilities, world class equipment, regulatory compliant software and hardware and secured networks.
Bioanalytical Research Unit
Synergen Bio has a state-of-the-art GLP compliant Bioanalytical Laboratory equipped to cater a variety of Bioanalytical
Research needs and equipped with most advanced LC-MS/MS systems for carrying out method development and validation and
regulated sample bioanalysis.
Synergen Bio supports sample preparation laboratory equipped with all typical laboratory equipment necessary for all the
bioanalysis work.